The Biomedical Device Laboratory has capital equipment and in-house characterization instrumentation to thoroughly characterize synthesized materials and devices. Our synthesized materials undergo mechanical, thermal, spectroscopic, and microscopic analysis. Following synthesis, the materials are used to fabricate medical devices that undergo a range of testing, including mechanical testing, in vitro testing in silicone models, and in vivo testing in animal models.
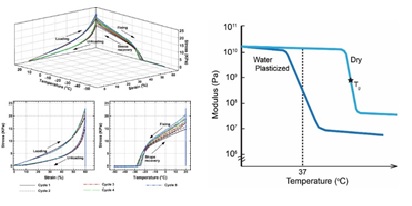
Polymer characterization is focused on mechanical, thermal, spectroscopic, and/or visual analysis. Mechanical analysis of SMPs includes tensile and compression testing to failure using Instron or MTS tensile testers. Thermal and thermomechanical analysis is performed using (1) dynamic mechanical analysis(DMA) to gain insight into the viscous and elastic nature of SMPs, (2) differential scanning calorimetry (DSC) to determine transition temperatures based on heat flow, and (3) thermogravimetric analysis (TGA) to assess thermal degradation temperatures. Spectroscopic analysis is typically performed using Fourier transform infrared (FTIR) spectroscopy , although other techniques can be utilized. Visual analysis includes light microscopy and scanning electron microscopy (SEM) to examine porous SMP structures. Other characterization methods include hydrophobicity measurements using goniometry, film thickness using elipsometry, mass loss using gravimetric analysis, and viscosity measurements using rheometry. These characterization techniques are used to assess SMP shape recovery properties to determine their suitability for incorporation into devices.
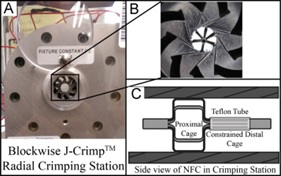
Devices made in the Biomedical Device Laboratory undergo extensive characterization to demonstrate safety and efficacy, a prerequisite for FDA-approval. Efforts include mechanical testing, in vitro flow studies with anatomical models, clinician feedback, fluoroscopic-guided device delivery, computational modeling, fluid dynamic characterization, and in vivo animal studies.
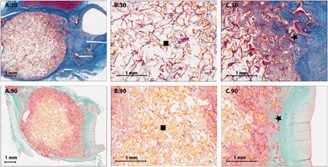
The Biomedical Device Laboratory performs cell studies for synthesized materials to assess in vitro cytocompatibility and cell material interactions. Animal model studies are utilized to measure safety and efficacy of medical devices. With the combined expertise of state and national collaborators, our devices are evaluated with a focus on future commercialization and incorporation into clinical use.